Which of the Following Compounds Is the Strongest Bronsted Acid
Hence c phenol is the strongest bronsted acid. Which of the following compounds is the strongest acid.

Solved Which Of The Following Compounds Is The Strongest Chegg Com
H2S is the stronger acid because there is a longer bond length between the sulfur and hydrogen.
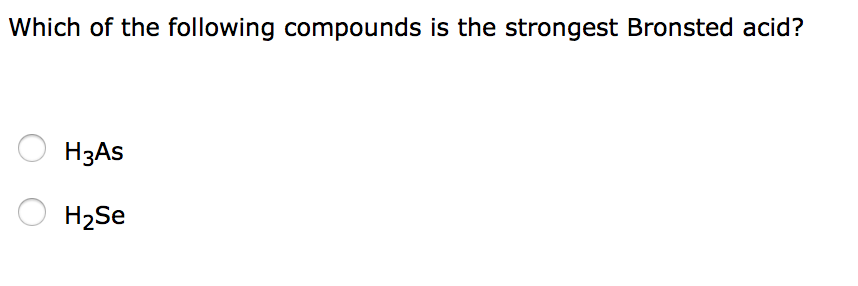
. And if you want to measure the strength of bronsted acid then. Hence conjugate base of c is more stable than d. Among HClO3 HClO2 and HOCl Cl has the highest oxidation state of in HClO3 and hence HClO3 is the strongest acid.
Stability of conjugate basestrength of acid. AC4H4N BSO42 CCH33N Write the formulas of the conjugate bases of the following Brønsted-Lowry acids. B HC CH is the strongest acid and has the shortest C-H bond distance.
A HC CH is the weakest acid and has the longest C-H bond distance. CH 2 FCH 2 CO 2 H. ACH3COOH bFCH2COOH cClCH2COOH dBrCH2COOH.
D CH2 CH2 is the strongest acid and has the shortest C-H bond distance. Acids Bases and Salts Of the following compounds which one is Of the following compounds which one is. Which of the following compounds is the strongest Bronsted acid.
Which of the following compounds is the strongest Bronsted acid. And coming to your question The worlds strongest acid at least a million times more potent than concentrated sulphuric acid is CARBORANE ACID. 1 CH2FCH2CO2H and 2.
CH 2 BrCH 2 CO 2 H. Which of the following compounds is the strongest Bronsted acid. Which of the following compounds is the strongest Bronsted acid.
In aqueous solution classify these compounds as strong. AHCN BCH32NH2 CH2SO4. HCl is the stronger acid because chlorine has a greater electronegativity than sulfur.
Of the following compounds which one is the strongest Bronsted acid in an aqueous solution - Of the following compounds which one is the strongest Bronsted acid in an aqueous solution - Getting Image Please Wait. Chemistry - Acid and Bases. Experts are tested by Chegg as specialists in their subject area.
Comparing c and d phenoxide ion c is more stable because the negative charge is delocalised in the benzene ring. Of the following compounds which one is the strongest Bronsted acid in aqueous solution. What is the conjugate base of H 2 O.
Higher be the oxidation number of central atom in oxo-acids more strongly it behave as Bronsted-acidOxidation number for Cl in HClO3 5Oxidation number for Cl in HClO2 3Oxidation number for Cl in HClO 1Oxidation number for Cl in HBrO 1Hence HClO3 is the strongest Bronsted acid in aqueous solution. Which of the following compounds is the strongest Bronsted acid. H 4 Ge vs H 3 As.
CH 2 BrCH 2 CO 2 H. Correct option is D A Bronsted acid is a species that gives a proton in aqueous solutions. Who are the experts.
Among the given compounds phenol is the only acid as the phenoxide ion formed after a proton is removed is stabilized by resonance. Hence it is the strongest Bronsted acid. Which of the following compounds is the strongest bronsted acid.
Which of the following compound is the strongest Bronsted acid. C CH3-CH3 is the strongest acid and has the longest C-H bond distance. Which of the following compounds is the strongest Bronsted acid.
Acids Bases and Salts. E None of the choices is correct. Of the following compounds which one is the strongest Bronsted acid in an aqueous solution.
CH 2 FCH 2 CO 2 H. See the answer See the answer done loading. If the corresponding conjugate base is weak then the bronsted acid is strong and vice-versa.
H 3 O vs CH 4. Which of the following compounds is the strongest Bronsted acid. Bronsted acid- conjugate base proton.
Which Bronsted acid HCl or H2Saq is the stronger acid and why.
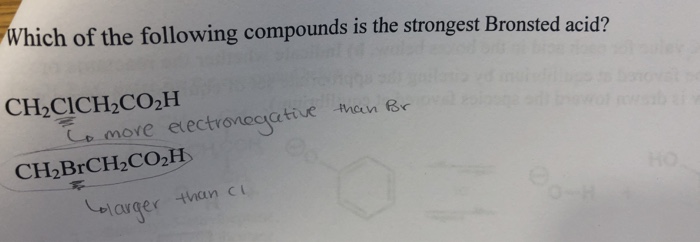
Solved Which Of The Following Compounds Is The Strongest Chegg Com
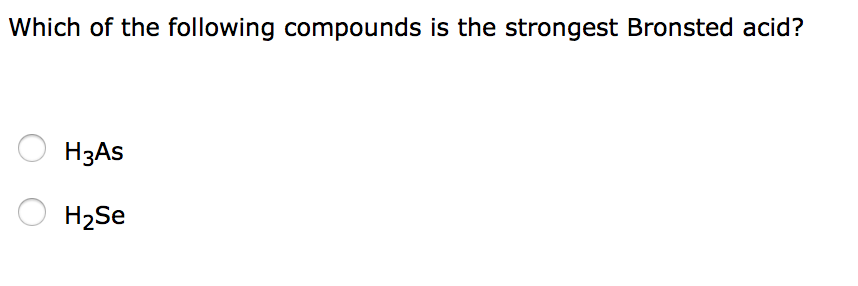
Solved Which Of The Following Compounds Is The Strongest Chegg Com
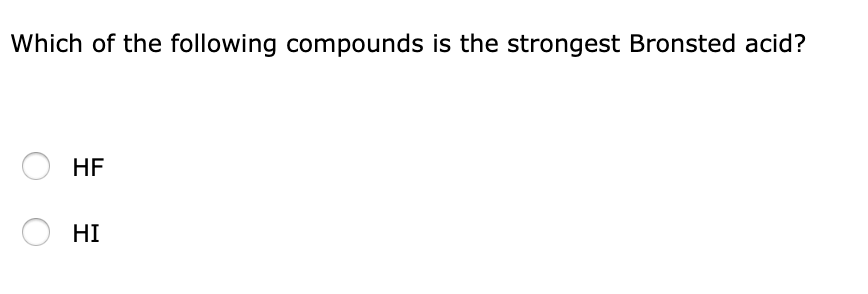
Solved What Is The Conjugate Base Of O2 H402 2 2 Ho H30 Chegg Com
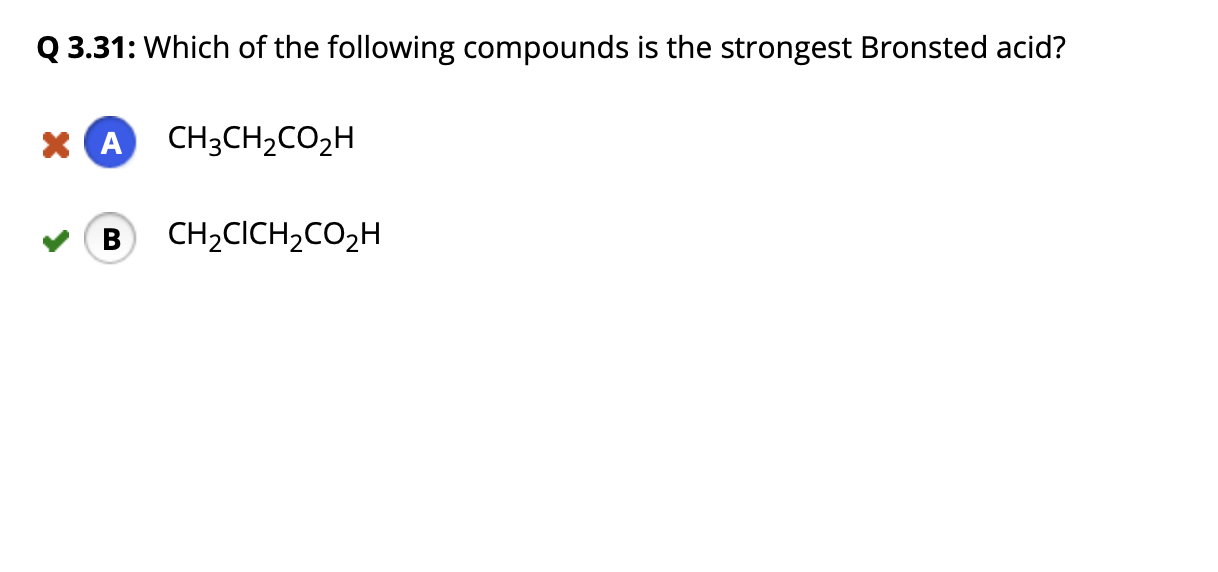
Solved Which Of The Following Compounds Is The Strongest Chegg Com
Comments
Post a Comment